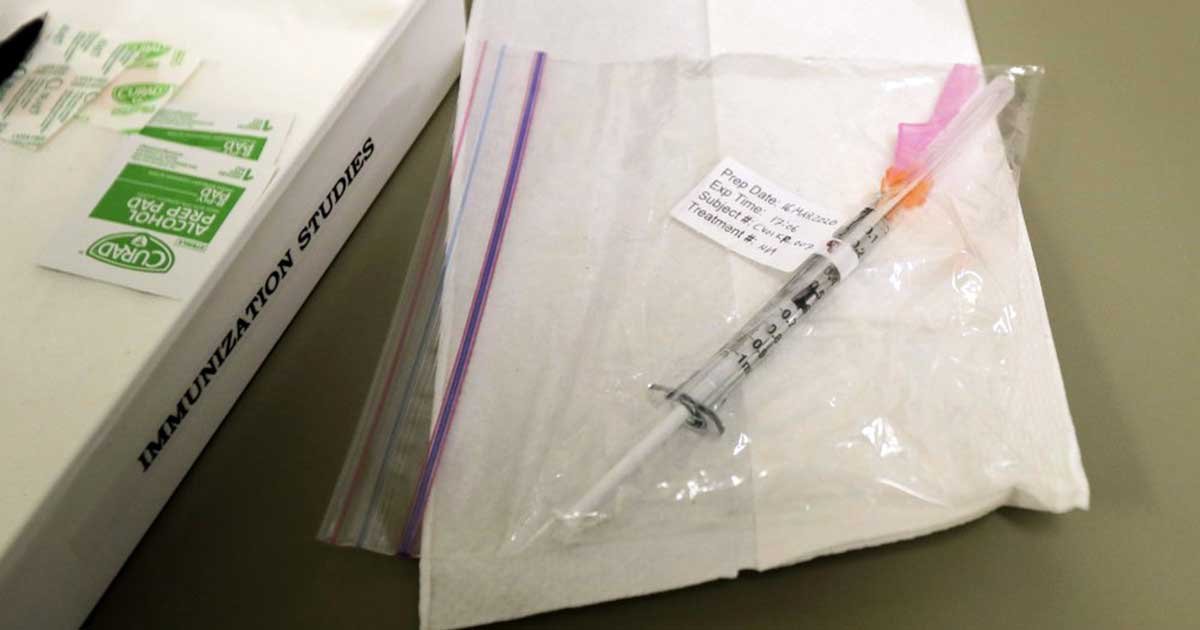
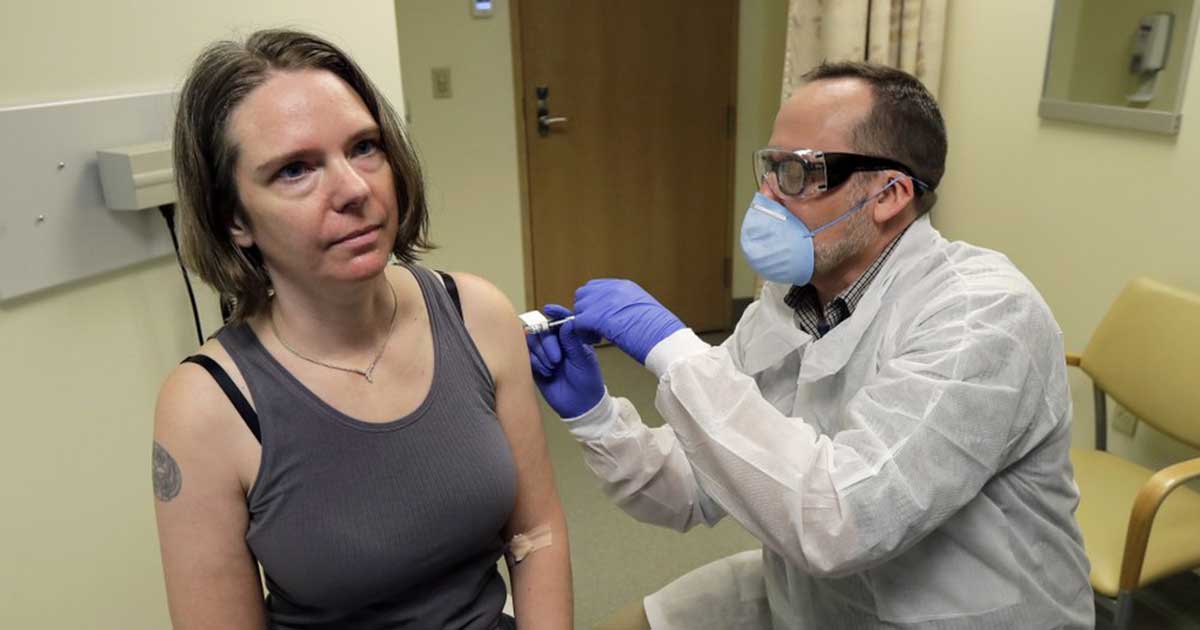
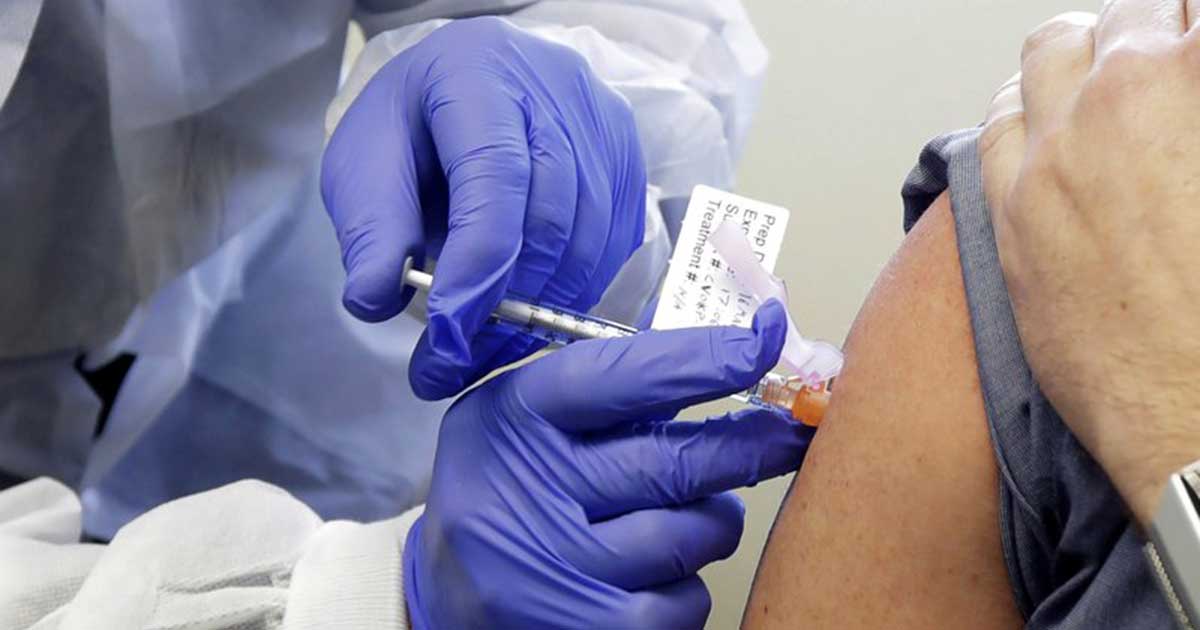
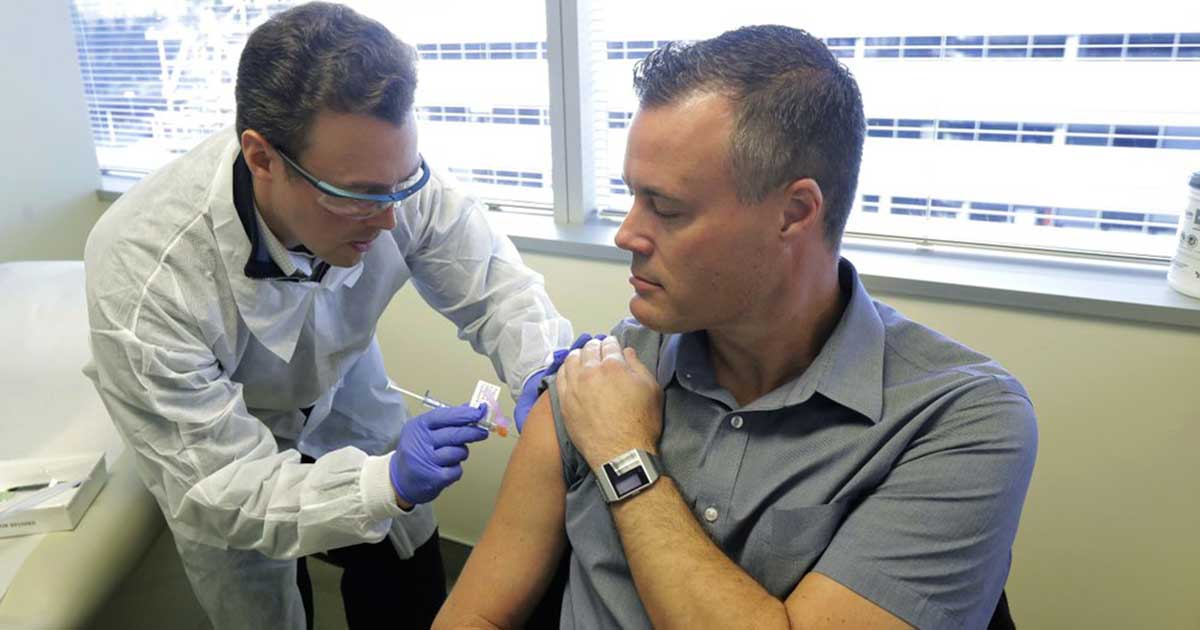
The first Covid-19 vaccine tested in the U.
S. is scheduled move into a much larger late-stage trial around July 27, after all patients tested in an initial safety trial produced antibodies against the coronavirus.
In an interview, the U.S. government’s top infectious disease expert, Dr. Anthony Fauci exclaimed, “No matter how you slice this, this is good news.”
Developed by National Institutes of Health and Moderna Inc., the experimental vaccine will move to a 30,000-person study to gather evidence that the shots really are strong enough to protect against the coronavirus.
Initial testing done in March was comprised of 45 volunteers, and all of them developed what are called neutralizing antibodies in their bloodstream.
According to the report published by the New England Journal of Medicine, these antibodies were key to blocking the infection at levels comparable to those found in people who survived COVID-19.
“This is an essential building block that is needed to move forward with the trials that could actually determine whether the vaccine does protect against infection,” said lead researcher Dr. Lisa Jackson of the Kaiser Permanente Washington Research Institute in Seattle.
The findings were considered an important early step in testing, although stimulating production of neutralizing antibodies does not prove a vaccine will be effective.
However, researchers from the National Institute of Allergy and Infectious Diseases reported that a number of patients in the trial experienced side effects.
Some of the side effects reported were fatigue, headache, chills, fever and pain at the injection site.
For participants whose reactions were more severe, they were given the highest dose – which researchers do not plan to pursue.
Dr. William Schaffner of Vanderbilt University Medical Center, a vaccine expert who was not involved with the study, shared his opinion to the study, citing that the side effects were a “small price to pay for protection against COVID.”