U.
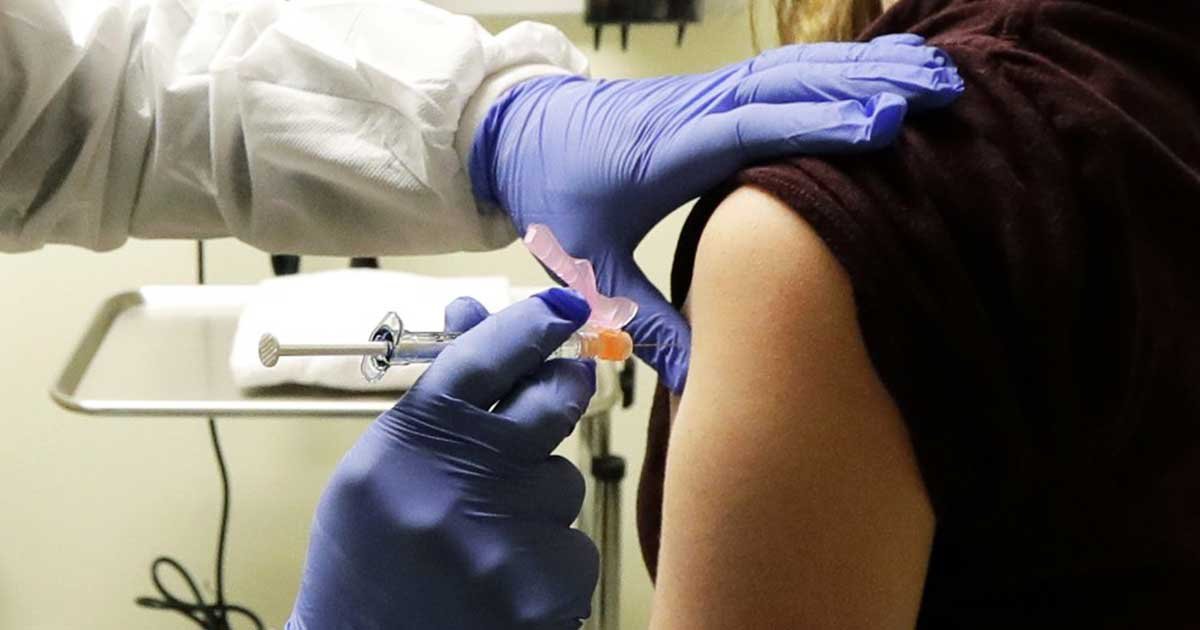
S. researchers administered the first shots of an experimental coronavirus vaccine on Monday, in an effort to create a protection against the rapidly surging pandemic.
Kaiser Permanente Washington Research Institute in Seattle began by administering the trial vaccine to four healthy volunteers as part of the first-stage study of a potential COVID-19 vaccine.
Kaiser Permanente study leader Dr. Lisa Jackson said in an interview, “We’re team coronavirus now. Everyone wants to do what they can in this emergency.”
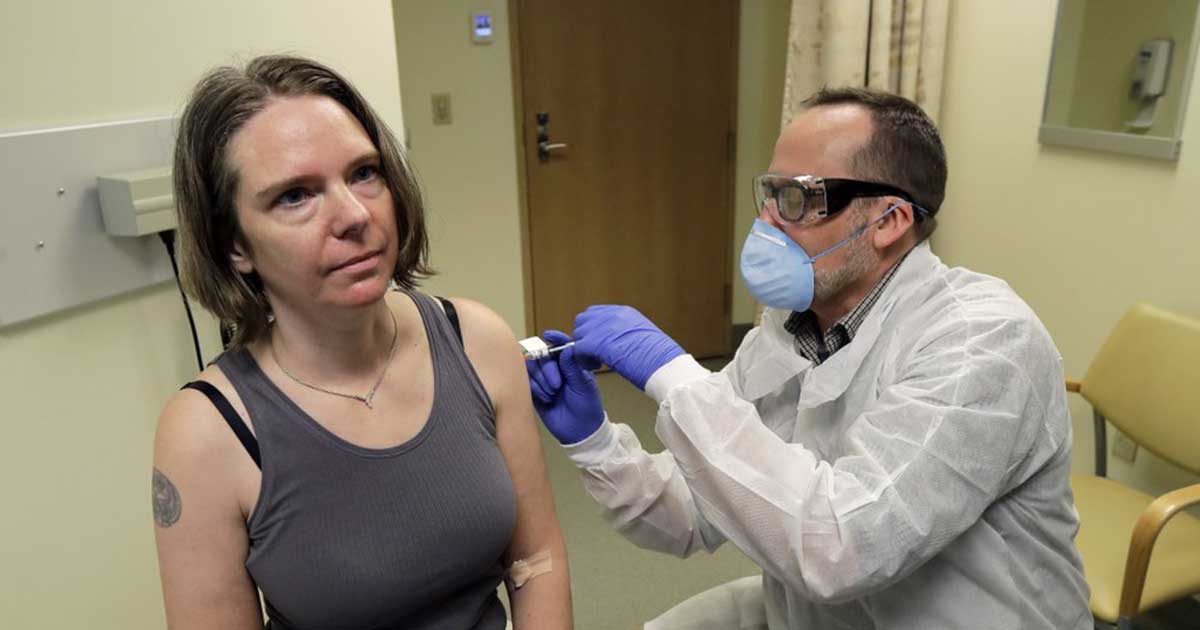
Jennifer Haller, a 43-year-old mother and an operations manager from Seattle is one of the four volunteers who have received the experimental vaccine.
“We all feel so helpless. This is an amazing opportunity for me to do something,” she said.
She left the exam room after the injection, and said with a big smile, “I’m feeling great.”
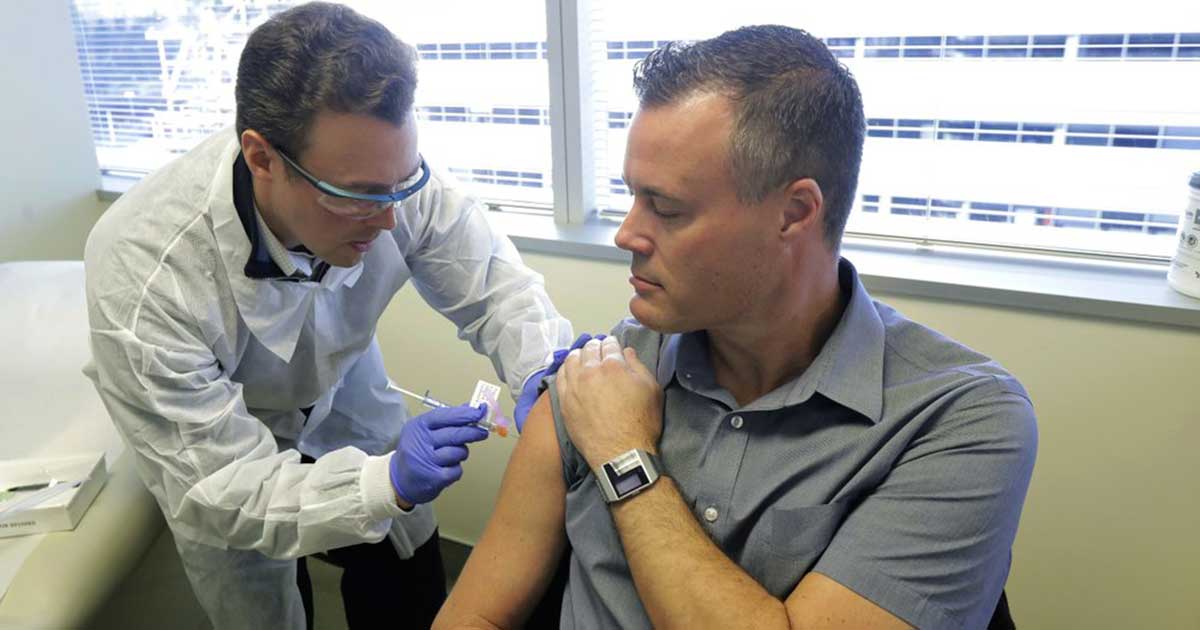
Neal Browning, a 46-year-old Microsoft network engineer from Bothell, Washington said his young daughters are proud that he volunteered.
He said, “Every parent wants their children to look up to them.”
Monday marked the beginning of a series of studies needed to see if the shots are safe and would work.
However, Dr. Anthony Fauci of the U.S. National Institutes of Health said that even if the research goes well, the vaccine would not be available for widespread use for 12 to 18 months time.
Fauci noted that it has been 65 days since Chinese scientists shared the virus’ genetic sequence, and he believes that was a record for developing an experimental vaccine.
Code-named mRNA-1273, this experimental vaccine was developed by the NIH and Massachusetts-based biotechnology company Moderna Inc.
This is not the only potential vaccine under study, as dozens of research groups race to create a vaccine against COVID-19.
Inovio Pharmaceuticals made another possible candidate, and is expected to conduct their own safety study in the U.S., China, and South Korea next month.