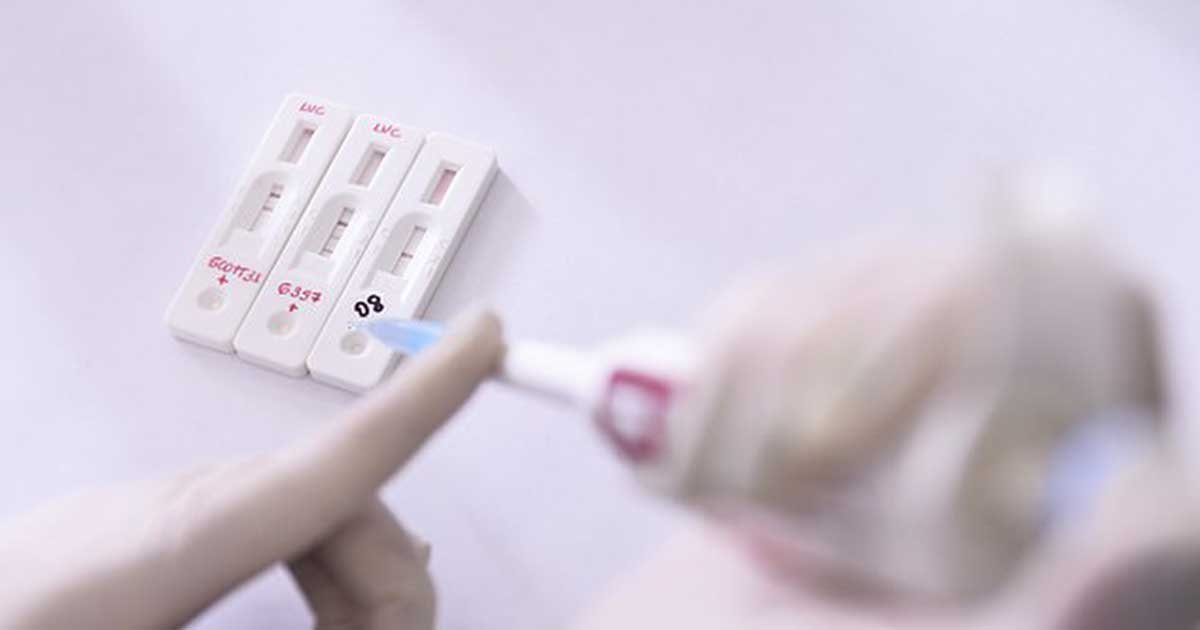
The Spanish government demands refund as the 640,000 antigen coronavirus tests bought from China after health authorities found the kits to be faulty.
The purchase was made via a Spanish distributor from a Chinese company called Bioeasy, and the said testing kits are supposed to replace the first batch which that did not work, as the tests do not have the sensitivity requirement to accurately detect the virus.
The Carlos III Health Institute in Madrid analyzed the sample kits, which uses a fluorescence method, provided by the Chinese company, and saw that they inadequately sensitive.
The government has decided to cancel the entire order, and the Health Ministry confirmed that they are in the process of claiming a refund.
The ministry has not announced who was the Spanish intermediary and how much the order from the Chinese company costs, nor have they published purchasing contracts for health materials amid the coronavirus pandemic.
This move goes against the report issued by the Public Procurement Advisory Board, a branch under the Finance Ministry, which requires them to do so.
The Spanish government also decided to abandon its plan to use rapid tests and would focus on tests that look for antibodies in recovered people, and in another, more expensive form of test that looks for the virus in people’s DNA.
The Chinese Embassy in Spain made an announcement on Twitter after the country received the first batch, and declares that Bioeasy does not have a license to sell its products from China’s National Medical Products Administration.
They also confirmed that the Chinese Ministry of Commerce did not include Bioeasy on the list of manufacturers they recommended to Spain, and the medical supplies donations by China did not include Bioeasy products.
The Spanish government, however, points out that Bioeasy had permission to export to the EU, and bought the tests from a Spanish distributor and not directly from the Chinese company.