A California biotech company announced positive results from their strict drug trial in hopes to fight COVID-19.
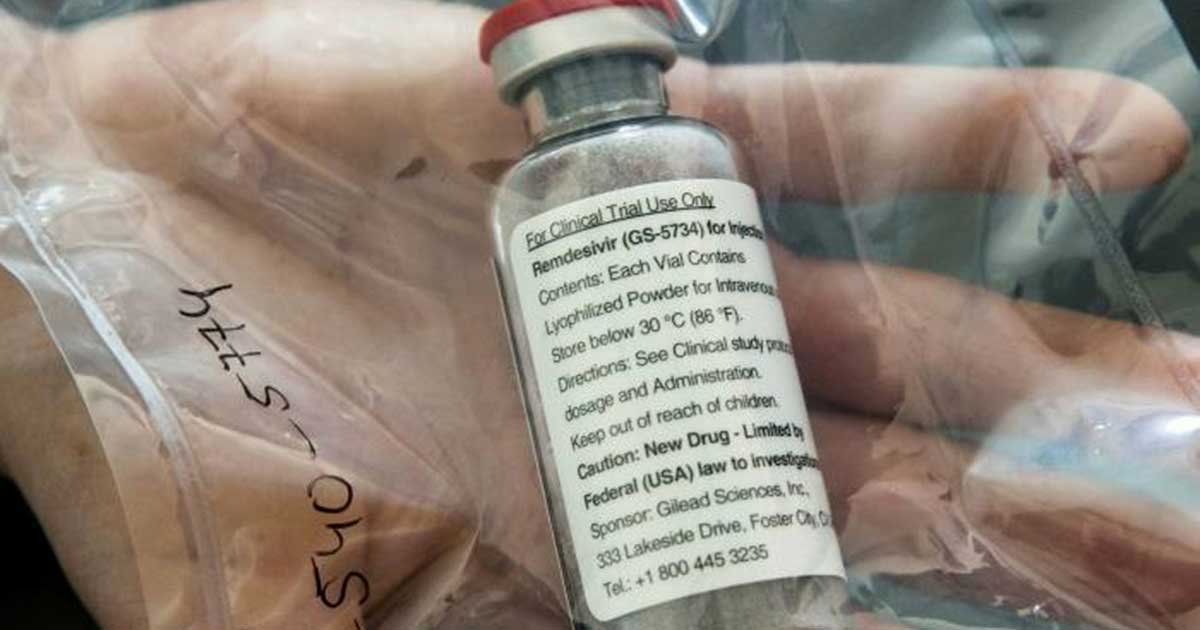
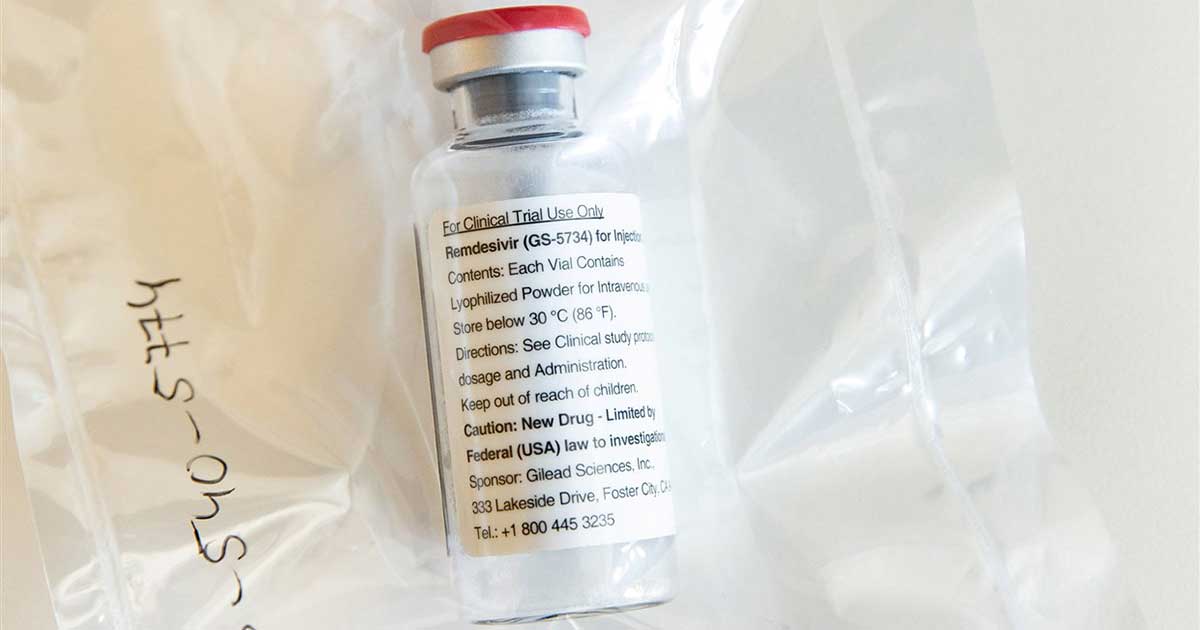
Remdesivir, an antiviral medication developed by Gilead Sciences, would be the first treatment to pass a trial against the novel coronavirus.
The treatment could have a significant effect on the pandemic, as health officials worldwide estimate that a vaccine will likely be available at least a year from now.
Spearheaded by the National Institutes of Health, the goal of the study was measure how long it takes for patients to recover, and pitted Remdesivir against usual care in about 800 confined coronavirus patients across the globe.
According to the biotech company’s website, the study comprises of evaluating hospitalized patients with severe COVID-19 manifestations, dividing them into two groups – one receiving 5-day dosing durations and the other receiving 10-day dosing durations.
Their statement says, “The study demonstrated that patients receiving a 10-day treatment course of Remdesivir achieved similar improvement in clinical status compared with those taking a 5-day treatment course.”
Chief Medical Officer Dr.
Merdad Parsey added, “Unlike traditional drug development, we are attempting to evaluate an investigational agent alongside an evolving global pandemic. Multiple concurrent studies are helping inform whether Remdesivir is a safe and effective treatment for COVID-19 and how to best utilize the drug.
”
Remdesivir is one of the many treatments being tested against COVID-19, and so far, the most promising.
The antiviral drug is administered through an IV and its design includes interfering with the virus’ ability to copy its genetic material.
Previous animal testing against SARS and MERS proved the drug’s ability to help prevent infection and reduce the severity of the symptoms, as long as it is administered early enough in the course of the illness.
However, Remdesivir is neither licensed or approved globally, and its safety and effectivity has not been established.