Australian researchers assert that finding a cure for coronavirus is “within control.
”
According to Professor David Paterson, who oversees the research at the Royal Brisbane and Women’s Hospital, two drugs have halted the deadly COVID-19 virus in its laboratory test tracks.
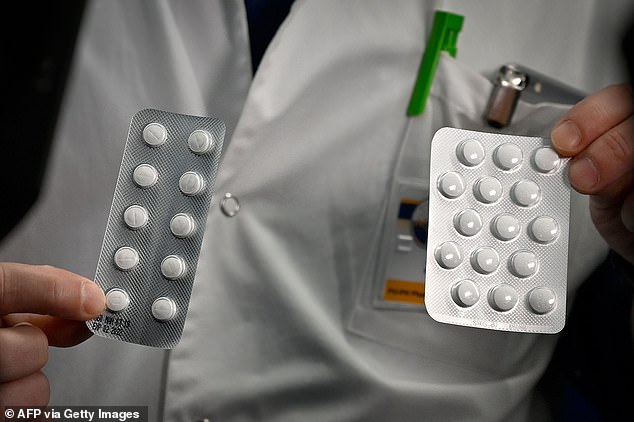
Chloroquine, an anti-malarial drug, and a hybrid of lopinavir / ritonavir that suppresses HIV, both have recently shown favorable results in human research and caused the virus to’ vanish’ in infected individuals.
That medicines are now being studied as doctors and physicians race all across the world to try to discover a vaccine, cure or therapy for the deadly virus.
These drugs are safe for use in Australia, one treating malaria, the other curing HIV and both killing the virus successfully in test tube tests.
“These medications have proved to be effective, they are still available in Australia and we hope they can kill the virus and have a cure on the basis of laboratory testing,” the renowned Infectious Diseases physician told.
Professor Paterson said that considering the medications as a potential ‘ known treatment’ for the deadly lung infection would be no wrong.
He clarified that it contributed to the’ disappearance of the virus’ when the HIV drug lopinavir / ritonavir was given to patients infected with coronavirus in Australia.
While the therapy was successful in a handful of cases, no standardized research like what should be required to develop a new medication has been carried out, Professor Paterson said.
He further continued, What we want to do at the moment is a massive clinical trial across Australia, looking at 50 hospitals, and what we’re trying to compare is one drug, versus another drug, versus the combination of the two drugs,’.
This is an antiviral drug that people diagnosed with HIV will take twice a day to minimize levels of the virus that circulates in the body.
It is a class of drugs called a protease inhibitor that works by preventing viruses from the use of a protease enzyme, which is essential for them to grow. Excluding protease viruses, can’t produce the fully developed clones they need to be able to infect other healthy cells, and they can’t transmit the infection.
Kaletra is authorized to use in the United States, Europe and Australia, and its maker, AbbVie, has since contributed drug supplies to China, the United Kingdom and the World Health Organization authorities. It is a separate formulation to the PREP drug that has recently been approved in the UK for HIV prevention.
However, Chloroquine is also commonly used by travellers as an antimalarial and is also approved in the UK to be used on people with rheumatoid arthritis or lupus.
In addition to the Royal Brisbane and Women’s Hospital, Professor Paterson, an infectious disease specialist, has launched a fundraising campaign to raise funds to support the clinical trials.
It follows as a senior US official announced that today, humans are going to launch trials of an experimental coronavirus vaccine.
Forty-five Seattle participants–currently devastated by an epidemic–will receive the jab to check if it’s secure.
The World Health Organization reports 35 new vaccines are under production, including one that the US government has co-developed.