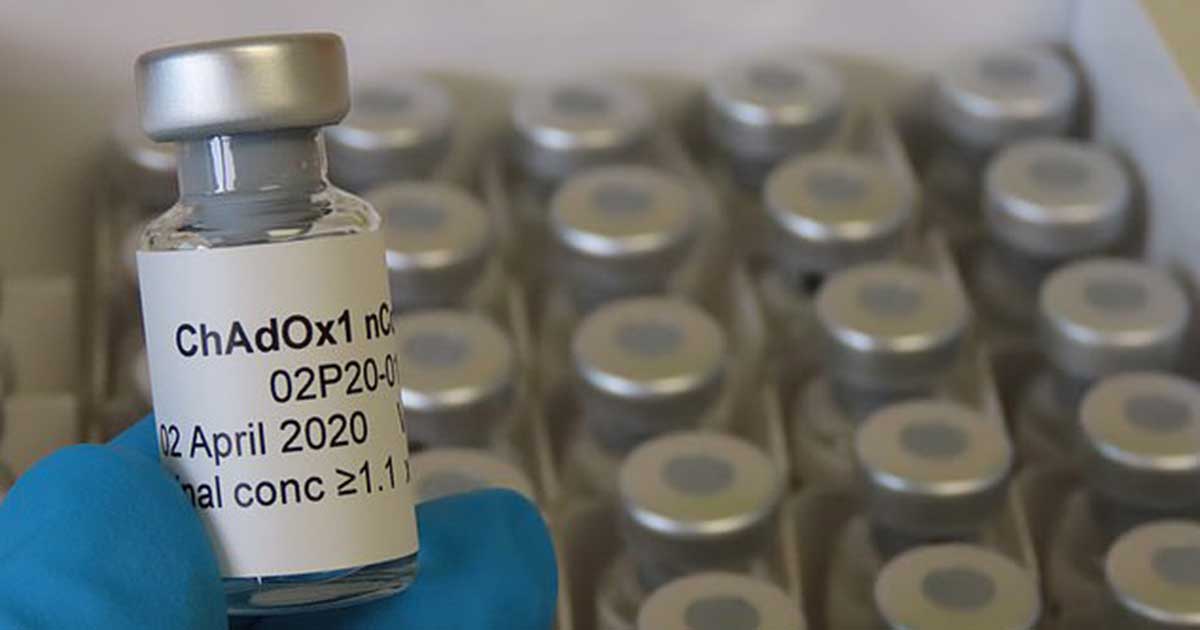
As scientists, researchers, and doctors around the world are racing to develop an effective treatment against COVID-19, Oxford University has reached the stage where they are to begin human trials for their coronavirus vaccine.
After months of struggling with the pandemic, the trial of the potential coronavirus vaccine is scheduled to begin next week.
It is estimated that the vaccine may be released for emergency use by autumn depending on the results of the trial.
The researches from the university have made trials on several animal species and yielded promising results, making it one of the many potential candidates being developed across the globe.
According to the World Health Organization, about 70 vaccines are currently in development from various parts of the world.
The Oxford researches are set to collaborate with a group of researchers in the United States and China as they start with the human trials.
Reports say the trials shall be conducted on 510 people between 18 to 55 years old.
Lead researcher Adrian Hill said, “We are going into human trials next week. We have tested the vaccine in several different animal species. We have taken a fairly cautious approach, but a rapid one to assess the vaccine that we are developing.”
The Professor of Vaccinology at the university, Sarah Gilbert, believes that there is an 80 percent chance that the trials will be a success, stating in an interview, “The prospects are very good, but it is clearly not completely certain.”
She added, “By the time we have all the approvals for the vaccine ready, we should have a good pool of volunteers to draw from and we should be able to get going quite quickly.”
Professor Gilbert stressed that Oxford University is not looking to make money out of the vaccine, and that they are concentrating on making it available for public health use.